This immediately will lead us to conclude that hydration. Carbonate is a doubly charged negative ion that should form strong electrostatic bonds with metal ions - the doubly charged calcium ion Ca2 should form strong ionic bonds and indeed it does.
 Solubility Of Calcium Carbonate Lime Scale In Water As A Function Of Ph Download Scientific Diagram
Solubility Of Calcium Carbonate Lime Scale In Water As A Function Of Ph Download Scientific Diagram
Deposits of calcium carbonate frequently found on potspans and kettles in areas where the water is hard can be removed by treating the utensils with vinegar which contains the weak acid acetic acid.
Calcium carbonate soluble in water. Calcium carbonate and bicarbonate are of low solubility and so cannot be concentrated indefinitely in cooling systems. These compounds are what you will find in supplements and include calcium carbonate calcium phosphate calcium lactate and calcium citrate. Surface water is somewhat acidic in nature because the CO₂ of air dissolves in rain and produce carbonic acid H₂CO₃.
Water-soluble calcium is a source of available calcium. The rock is limestone which is usually composed of pure calcium carbonate. Carbonate or gypsum calcium sulfate Hodges 2010.
Although calcium citrate does have a higher solubility than CaCO3 in water there is little difference when the pH is controlled at pH 75. Calcium carbonate appears as white odorless powder or colorless crystals. The fact is calcium carbonate is insoluble and calcium bicarbonate is soluble in water.
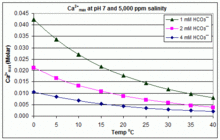
The partial pressure of CO2 also played a role in calcium carbonate solubility depressing the solubility at pH 75. Acidic water greatly enhances the solubility of calcium carbonate and it doesnt even need to be highly acidic. Ground calcium carbonate CAS.
One contribution to the reason that calcium carbonate precipitates on heaters is simply that calcium carbonate in seawater is slightly less soluble as the temperature rises. These vitamins need dietary fat in order to be better absorbed in the small intestines. Calcium carbonate does not dissolve in water is not soluble in water but like all carbonates it reacts with acids.
This forms because calcium carbonate dissolves. For example in the natural. In fact most carbonates are insoluble in water the exceptions are.
Since they are alkaline an increase in their concentration also results in a rise of pH in the cooling water. As physical scientists however we seek actual data and I confess that I cannot find an appropriate table. Water-soluble calcium WCA is an alternative to these commercial sources of calcium.
Occurs extensive in rocks world-wide. Calcium carbonate is unusual in that its solubility increases as the temperature of the water decreases. Practically insoluble in water.
Calcium carbonate has a very low solubility in pure water 15 mgL at 25C but in rainwater saturated with carbon dioxide its solubility increases due to the formation of more soluble calcium bicarbonate. The water-soluble vitamins are the B vitamins and vitamin C. Rain or river water that come into contact with the atmosphere absorb the C O X 2 as.
Calcium carbonate is insoluble in water. Fat-soluble vitamins dissolve in fat not water. This fact sheet ad-dresses frequently asked questions about making WCA and its use in Natural Farming.
Calcium carbonate is only slightly soluble in water. 1317-65-3 results directly from the mining of limestone. Since calcium carbonate is already supersaturated the effect is that when the water is warmed the supersaturation of calcium carbonate rises making precipitation more likely.
Calcium carbonate CaCO₃ is nothing but limestoneCaCO₃ is not soluble in water but it does dissolve a solvent which is weakly acidic. Langelier defined the relation between the calcium alkalinity and pH of saturation in mathematical terms.