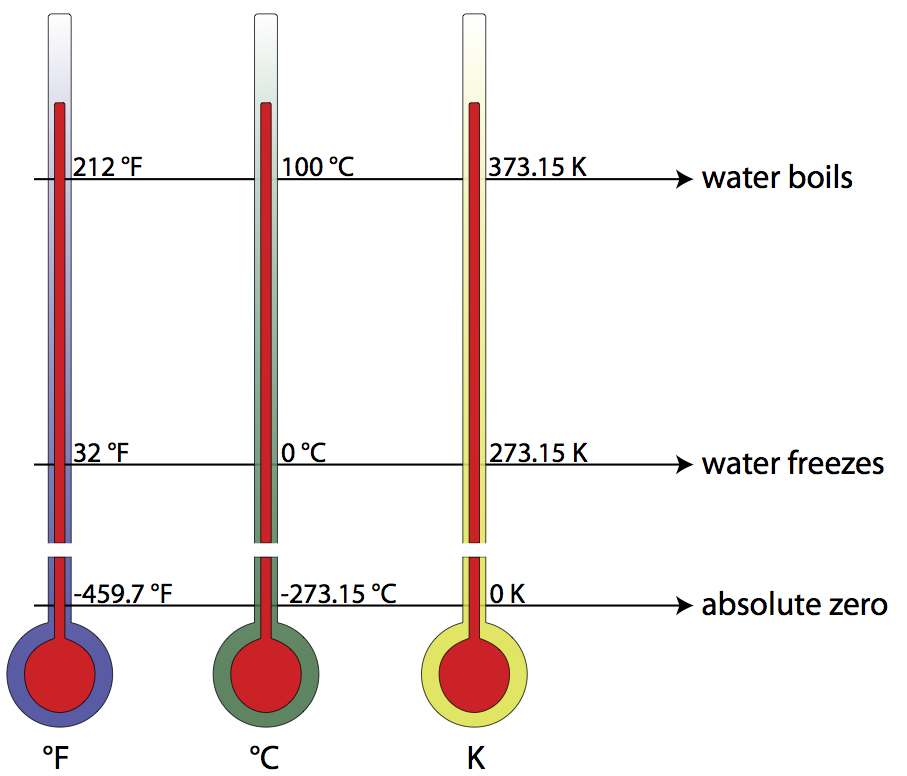
The boiling point of water in Fahrenheit is 212 degree F and in Celsius it is 100 degree Celsius. What is the boiling temperature of water in Fahrenheit.
 Temperature And Temperature Scales Chemistry For Non Majors
Temperature And Temperature Scales Chemistry For Non Majors
In the Fahrenheit scale water boils at 212 degrees.
Boiling temperature of water in fahrenheit. Add 32 to this number. Boiling Point - Celsius. Water freezes at zero degree celsius and it boils at hundred degree celsius.
The IUPAC recommended standard boiling point of water at a standard pressure of 100 kPa 1 bar is. 22 Zeilen Boiling Point - Fahrenheit. The boiling temperature of water is somewhere around 110 degrees Fahrenheit.
See answers 1 Ask for details. The melting point of ice is for this exercise at 32 F and the boiling point of water is at 212 F. Basically the task is.
At sea level vapour pressure is equal to the atmospheric pressure at 100 C and so this is the temperature at which water boils. 0 m 212 ºF. Because the mercury thermometer was more accurate Fahrenheit decided to expand the Roemer scale by multiplying its values by four.
Similarly the freezing point of water at sea level is a constant value - 0C or 32F. So to convert in fahrenheit just multiply the C temperature by 18. The Fahrenheit is a scale which is used to measures temperature.
It is always the same - 100C or 212F. -12 C or 165 F and the output should be what state water is in at the entered temperature. The temperature of boiling water is.
There are two conventions regarding the standard boiling point of water. The exact freezing and boiling points of plain water minus the salt were marked at 32 and 212 degrees Fahrenheit respectively. By signing up youll get thousands of step-by-step solutions to your homework.
Boiling point of water. The normal boiling point is 9997 C 2119 F at a pressure of 1 atm ie 101325 kPa. 5 points shyyyboss Asked 09082019.
What is the boiling temperature of water in Fahrenheit. Actually the formula for boiling point uses this value as the basis of calculations. Now the Fahrenheit scale was developed based on the temperature measurement of the atmospheric air.
I should input a temperature value followed by a blank and the letter C for Celsius or F for Fahrenheit for example. Follow Report Log. Normal human body temperature was marked at 986.
F C 95 32. You dont have to use our boiling point at altitude calculator to determine the boiling point of water at sea level. Hence this scale was developed keeping the freezing and boiling points of the water as standard from 0 to 100.
The simple answer to this question is that the boiling point of water is 100 C or 212 F at 1 atmosphere of pressure sea level. Get the answers you need now. Fahrenheit is a temperature scale that bases the boiling point of water at 212 and the freezing point at 32.
Boiling point of water is 100 C. At which temperature fahrenheit does water boil. Fahrenheit temperature scale was proposed by a German physicist Daniel Gabriel Fahrenheit 1686-1736.
Water - Thermophysical Properties - Thermal properties of water - density freezing temperature boiling temperature latent heat of melting latent heat of evaporation critical temperature and more Water Boiling Points at Higher Pressure - Online calculator figures and tables showing boiling points of water at pressures ranging from 147 to 3200 psia 1 to 220. The boiling point of water depends on the atmospheric pressure which changes according to elevation. However the value is not a constant.
What is the tempreture of boiling water. When the vapour pressure reaches an equivalent value to the surrounding air pressure the liquid will boil. This the answer in F.
Click to see full answer. This was later reversed by Carolus Linnaeus or Carl Linnaeus a Swedish botanist physician and zoologist after Anders died.
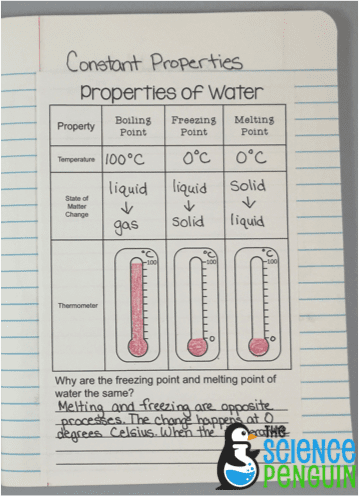 Constant Properties Of Water Boiling Point Melting Point And Freezing Point The Science Penguin
Constant Properties Of Water Boiling Point Melting Point And Freezing Point The Science Penguin
The red line shows the atmospheric pressure of the selected planet.

Boiling and freezing point of water. Water boils if the surrounding gases in the air have a high pressure. The boiling point of water varies with atmospheric pressure. A solution boils at a slightly higher temperature than the pure solvent.
Previously boiled water freezes faster than regular water Notwithstanding the previous explanation water at room temperature that was once boiled according to some experts should freeze faster because the dissolved oxygen has been removed. This week we will be identifying the boiling point of water and the freezing melting point of water. The change in the boiling point is calculated from.
As liquid matter boils it eventually vaporizes into a gas at the boiling point. Identify the boiling and freezingmelting points of water on the Celsius scale. For example the freezing point of pure water at standard atmospheric pressure or zero feet is 0C 32F while at 11km 6 miles above sea level it would only be 0001C higher.
At lower pressure or higher altitudes the boiling point is lower. On the Fahrenheit scale however freezing is 32 degrees and boiling 212. The freezing point describes the liquid to solid transition while the melting point is the temperature at which water goes from a solid ice to liquid water.
The freezing point or melting point of water is the temperature at which water changes phase from a liquid to a solid or vice versa. At 10000 feet above sea level the pressure of the atmosphere is only 526 mmHg. For example the boiling point of pure water at 10.
A mathematical equation is used to calculate the boiling point elevation or the freezing point depression. The boiling and freezing points of water enable the molecules to be very slow to boil or freeze this is important to the ecosystems living in water. A similar property of solutions is boiling point elevation.
Water has a boiling point of 100 C. The gas it usually turns into is called water vapor or steam. Textatm is 100texto textC while the boiling point of a 2.
Just as we can lower the boiling point we can also raise the boiling point of. Previously boiled water boils faster than regular water. Δ T b K b.
Under normal conditions when the pressure of the atmosphere is approximately 760 mmHg water boils at 100 o C. Because the freezing point of pure water is 0C the sucrose solution freezes at 068C. Scientists have found liquid water as cold as -40 degrees F in clouds and even cooled water down to -42 degrees F in the lab.
The boiling point elevation is the amount that the boiling point temperature increases compared to the original solvent. I cant tell you how surprised I was the first time I boiled water for my fifth graders. Not everyone has a vacuum chamber yet although they are getting quite inexpensive these days.
Boiling water is no big deal to us grown-ups because we see it almost every time we cook. The Fahrenheit scale defines the freezing point of water as 32F and the boiling point as 212F. The blue line shows the vapor pressure of water as a function of temperature.
The normal boiling point of water is 100 o C because this is the temperature at which the vapor pressure of water is 760 mmHg or 1 atm. The boiling point is the temperature at which the vapor pressure is equal to the atmospheric pressure. This Demonstration shows the boiling temperature of water on six planets all of which have different atmospheric pressures.
The meltingfreezing and boiling points change with pressure. The freezing point of water in degree Celsius is 0 C. Boiling point is the temperature at which something becomes a gas.
At at high altitudes the lower pressure makes the boiling point several degrees lower. This means that at 100 C water. At sea level pure water boils at 212 F 100C.
The Celsius scale sets the freezing point and boiling point of water at 0C and 100C respectively. Originally Anders Celsius assigned zero to stand for waters boiling point and 100 to stand for waters freezing point. Thats not always the case though.
The formula Degree Calsius C 27315 k 0 C 27315 27315k. In theory the two temperatures would be the same but. Then water gets to what is known as its boiling point.
The student is expected to. Raising the boiling point. If water was very easy to freeze or boil drastic changes in the environment and so in oceans.
For pure water the boiling point is 100 degrees Celsius 212 Fahrenheit at one atmosphere of pressure and the melting point is 0 degrees Celsius 32 degrees Fahrenheit at one atmosphere of pressure. Weve all been taught that water freezes at 32 degrees Fahrenheit 0 degrees Celsius 27315 Kelvin. Boiling Freezing and Pressure Altitude.
So 100ºC is now the boiling point of water while 0ºC is the freezing point of water. This may not be by a noticeable amount due to the volume change upon melting being much smaller than the volume change expansion when boiling.
In the case of the salt water one molecule contains two elements - Sodium Na and Chloride Cl. Water freezes at 32 degrees Fahrenheit 0 degrees Celsius but when a solute such as sugar is added the freezing point changes.
So Sucrose has a relative freezing point depression of.

Freezing point of sugar water. Temperature measures how much. This will give you the total concentration of particles dissolved. Multiply the original molality text m of the solution by the number of particles formed when the solution dissolves.
Some examples for grams of sugar added to 1 liter water. There are many ways in which sugar molecules can fit into this jumble. A 5 ww solution of cane sugar in water has freezing point of 271 K.
This link says the standard 41 solution starts freezing at 26-27 degrees F. The change in freezing point compared to pure water is calculated by the difference between the average freezing point and the freezing point of water which is 0 ºC. So to calculate the freezing point depression do.
For water Kf is 186Kkgmol and the molar mass of sugar sucrose is 342gmol. Sugar molecules dont fit well in that array. Td 186 342 mass of sugar in g mass of water in kg.
The freezing point of 5 ww glucose in water is. The average of the freezing point of the sugar solution of 27 mol kg would be. The effective freezing point of sugar water is somewhat below 0 C.
The reason the salt and sugar lowers the freezing point and the water takes longer to freeze is because the salt or sugar molecules interfere with the attraction between the water molecules and therefore interfere with the webbing of the water molecules which prevents the water from turning into ice when the water temperature lowers even further it eventually reaches a temperature where the water. If you have added 1 molecule of sugar to water the water will freeze later at -18 C 2876 Fahrenheit. Popsicles are essentially just frozen sugar water -- theres no real reason why sugar water wont freeze.
For pure water this happens at 32 degrees Fahrenheit and unlike most other solids ice expands and is actually less dense than water. The degree to which a sugar lowers the freezing point of water is called the Relative Freezing Point Depression. Slowly freezing the water where the ice forms on one side and grows to another will probably separate the sugar out much better than quick freezing with many nucleation sites.
Note that CaCl 2 is substantially more effective at lowering the freezing point of water because its solutions contain three ions per formula unit. This will be determined by gathering data on the freezing point of several sugar and water solutions. Because the freezing point of pure water is 0C the actual freezing points of the solutions are 22C and 30C respectively.
A water molecule consists of one oxygen atom and two hydrogen atoms. The rate of freezing may play a very important role. Why Does Sugar Affect the Freezing Point of Water.
The sugar solution is a nonelectrolyte so its i value is only 1. Just like Relative Sweetness Relative Freezing Point Depression is measured against Sucrose. Why does that make it harder for the ice to form lowering the freezing point.
The sugar in a popsicle may be predominantly between less. A 6040 solution of ethylene glycol and water has a freezing point of about -45 C. The vant Hoff factor will have a direct relationship with the freezing point depression.
Via R 5 ww 514 51 fish 271 K C 5 ww out Hich - 1 2692K 2 271 K 3 272 K 4 2672K 5 none of these HT 7 11th. The temperature at which a liquid turns into a solid is known as its freezing point. The higher total concentration will result in a higher boiling point and a lower freezing point.
Plain water freezes at 32 degrees F but when sugar or salt or other solutes are dissolved in it the freezing point gets lower. 2925 2 27 ºC. Sugar water freezes faster than salt water because salt has more molecules than sugar.
The sugar solutions will then be compared to a sodium chloride solution on account. So when you freeze sugar water or juice the sugar stays behind in the liquid water as the ice forms. Heres a clue- it turns out that the type of molecule dissolved in the water doesnt make nearly as much difference for the freezing.
The sugar molecules prevent the water from making hydrogen bonds which are required for solidity and the water has to become even colder before it reaches its freezing point. The freezing point depression constant of water is what needs to be found. Gl Td 10 0054 50 027 500 27.
Normally water freezes at 32F however when a substance is added Normally water freezes at 32F however when a substance is added. Frozen water ice is a regular array of molecules. The sugar molecules will depress the water freezing point further.
A strong salt solution has a freezing point of about -21 C. Freezing happens when the molecules of a liquid get so cold that they slow down enough to hook onto each other forming a solid crystal.
This immediately will lead us to conclude that hydration. Carbonate is a doubly charged negative ion that should form strong electrostatic bonds with metal ions - the doubly charged calcium ion Ca2 should form strong ionic bonds and indeed it does.
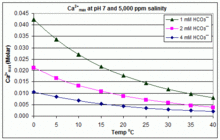
 Solubility Of Calcium Carbonate Lime Scale In Water As A Function Of Ph Download Scientific Diagram
Solubility Of Calcium Carbonate Lime Scale In Water As A Function Of Ph Download Scientific Diagram
Deposits of calcium carbonate frequently found on potspans and kettles in areas where the water is hard can be removed by treating the utensils with vinegar which contains the weak acid acetic acid.
Calcium carbonate soluble in water. Calcium carbonate and bicarbonate are of low solubility and so cannot be concentrated indefinitely in cooling systems. These compounds are what you will find in supplements and include calcium carbonate calcium phosphate calcium lactate and calcium citrate. Surface water is somewhat acidic in nature because the CO₂ of air dissolves in rain and produce carbonic acid H₂CO₃.
Water-soluble calcium is a source of available calcium. The rock is limestone which is usually composed of pure calcium carbonate. Carbonate or gypsum calcium sulfate Hodges 2010.
Although calcium citrate does have a higher solubility than CaCO3 in water there is little difference when the pH is controlled at pH 75. Calcium carbonate appears as white odorless powder or colorless crystals. The fact is calcium carbonate is insoluble and calcium bicarbonate is soluble in water.
The partial pressure of CO2 also played a role in calcium carbonate solubility depressing the solubility at pH 75. Acidic water greatly enhances the solubility of calcium carbonate and it doesnt even need to be highly acidic. Ground calcium carbonate CAS.
One contribution to the reason that calcium carbonate precipitates on heaters is simply that calcium carbonate in seawater is slightly less soluble as the temperature rises. These vitamins need dietary fat in order to be better absorbed in the small intestines. Calcium carbonate does not dissolve in water is not soluble in water but like all carbonates it reacts with acids.
This forms because calcium carbonate dissolves. For example in the natural. In fact most carbonates are insoluble in water the exceptions are.
Since they are alkaline an increase in their concentration also results in a rise of pH in the cooling water. As physical scientists however we seek actual data and I confess that I cannot find an appropriate table. Water-soluble calcium WCA is an alternative to these commercial sources of calcium.
Occurs extensive in rocks world-wide. Calcium carbonate is unusual in that its solubility increases as the temperature of the water decreases. Practically insoluble in water.
Calcium carbonate has a very low solubility in pure water 15 mgL at 25C but in rainwater saturated with carbon dioxide its solubility increases due to the formation of more soluble calcium bicarbonate. The water-soluble vitamins are the B vitamins and vitamin C. Rain or river water that come into contact with the atmosphere absorb the C O X 2 as.
Calcium carbonate is insoluble in water. Fat-soluble vitamins dissolve in fat not water. This fact sheet ad-dresses frequently asked questions about making WCA and its use in Natural Farming.
Calcium carbonate is only slightly soluble in water. 1317-65-3 results directly from the mining of limestone. Since calcium carbonate is already supersaturated the effect is that when the water is warmed the supersaturation of calcium carbonate rises making precipitation more likely.
Calcium carbonate CaCO₃ is nothing but limestoneCaCO₃ is not soluble in water but it does dissolve a solvent which is weakly acidic. Langelier defined the relation between the calcium alkalinity and pH of saturation in mathematical terms.
Freezing is slowed down during crystallization and temperatures remain constant until freezing is complete. The size of a one degree change in temperature is exactly the same in the Celsius and Kelvin scales so the freezing point of water is at a temperature of 27315 kelvins that is 27315 degrees above absolute zero.
The melting point of ice on Kelvin.

Freezing point of water in kelvin. The size of a one degree change in temperature is exactly the same in the Celsius and Kelvin scales so the freezing point of water is at a temperature of 27315 kelvins that is 27315 degrees above absolute zero. Kelvin to Celsius conversion. 0 k 27315C.
What is 1 Kelvin. Freezing point of water is 27315 K. Celsius conversely uses the point of reference of water freezing at the bottom of its scale and this doesnt accurately account for heat energy left in the water at this point 27315 K.
When measuring temperature the usual units are Celsius degree Celsius or Fahrenheit degree Fahrenheit. The size of a one degree change in temperature is exactly the same in the Celsius and Kelvin scales so the freezing point of water is at a temperature of 27315 kelvins that is 27315 degrees above absolute zero. 27315 K since the freezing point of water is 0OC and to convert celcius to kelvin you add 27315 and 027315 27315.
The temperature for the Kelvin scale. The freezing point of water on the Kelvin scale is 27315 K while the boiling point is 37315 K. Water boils at 3732 K Kelvin 100ºC Celsius or 212ºF Fahrenheit.
Ordinarily the freezing point of water and melting point is 0 C or 32 F. Absolute zero is 27315 C or 45967 F. People also ask what does water freeze at in Kelvin.
Hence by T K toC 27315. The freezingmelting point of water is about 27315 K at a pressure of 1 atmosphere. The temperature may be lower if supercooling occurs or if there are impurities present in the water which could cause freezing point depression to occur.
The melting point of water in kelvin. Thus zero degrees C the freezing point of water is exactly 27315 K. Under certain conditions water may remain a liquid as cold as -40 to -42F.
Then what is the purpose of the Kelvin temperature scale. On this scale the freezing point of water is 32 degrees Fahrenheit F and the boiling point 212 F. The boiling point of water in Kelvin is 373 K.
Find the value of 50F in Kelvin. The two most widely used measurements of temperature are Fahrenheit and Celsius. Find the value of 41F in Celsius.
The symbol of Kelvin is K. Boiling Point for water Celsius 100 degree Celsius. However when reporting temperatures in Kelvin we dont say degree Kelvin.
We know the exact freezing point of water in Celcius. Kelvin and centigradeCelsius are related in that the degree steps are identical but the K scale is offset by 27315 degrees. Temperature scale in which zero occurs at absolute zero and each degree equals one kelvin.
Instead these temperature units are thought of as kelvins. The size of a one degree change in temperature is exactly the same in the Celsius and Kelvin scales so the freezing point of water is at a temperature of 27315 kelvins that is 27315 degrees above absolute zero. The Celsius and Fahrenheit scales were both built around water either the freezing point the boiling point or some combination of water and a chemical.
Kelvin is a unit of measurement of temperature. The freezing point of water on the Kelvin scale is 27315 K while the boiling point is 37315 K. Additionally what is the melting point in Kelvin scale.
Boiling Freezing Melting point of water in kelvin 0F 1778C. Under certain conditions water may remain a liquid as cold as -40 to. What is Kelvin.
10C 27315 28315 k. Water freezes at 27315 K and boils at 37315 K. Absolute temperature using degrees the same size as those of the Fahrenheit scale in which the freezing point of water is 49169 and the boiling point of water is 67169.
1C 27315 27415 k. What is the Freezing Point of Water in Kelvin The freezing point of water in Kelvin is 273 K. The boiling point of water is 100oC.
T 10027315 37315K. Ordinarily the freezing point of water and melting point is 0 C or 32 F. Absolute zero is 27315 C or 45967 F.
Hereof what is the melting freezing point in Kelvin. The first was proposed in 1724 and is still the official scale in the United States.