Atomic number and mass number are always whole numbers because they are obtained by counting whole objects protons neutrons and electrons. The average mass of a chemical element expressed in atomic mass units.
Why Is The Word Average Used In The Definition Of Atomic Mass Quora
According to the definitions given atomic mass which is also called atomic weight is the measured total mass of an elements atom.
Define atomic number and atomic weight. The atomic weight of an element having more than one principal isotope is calculated both from the atomic masses of the isotopes and from the relative abundance of each isotope in nature. The Atomic weight is defined as the. Atomic Number and Atomic Weight The atomic weight or the relative atomic mass is the ratio of the average atomic mass of an atom to some reference standard.
When molecular mass expressed in gram is called gram molecular weight. The mass number reports the mass of the atoms. In learning chemistry the molecular mass or weight of the compound meaning the number that indicates how heavy a molecule of the compound is compared to one-twelfth of the carbon-12 isotope atomic number 12.
Atoms of different elements usually have different mass numbers. Atomic number has no units. Number of electrons in a neutral atom Mass Number.
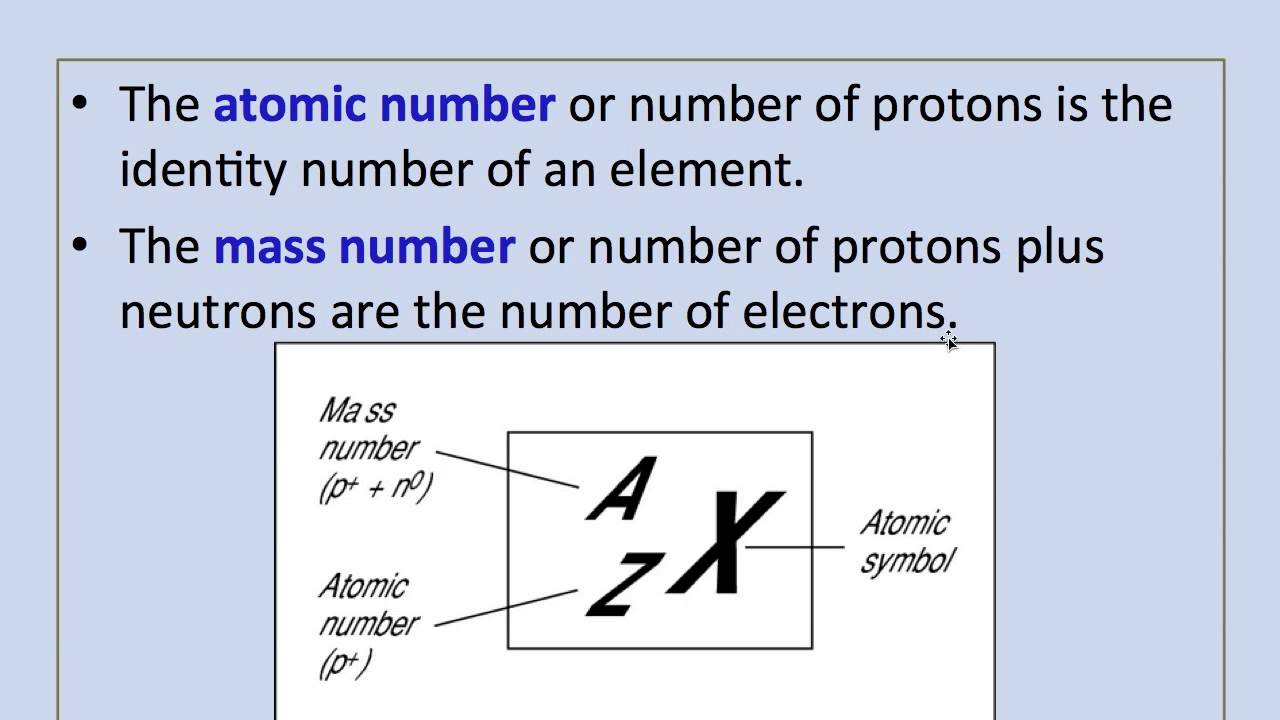
The mass number is a count of the total number of protons and neutrons in an atoms nucleus. Number of protons and neutrons combined in its nucleus. The mass number of an atom is its total number of protons and neutrons.
The atomic number of an atom is the number of protons in its nucleus ie. Whereas atomic number is nothing but the total number of neutrons and protons in the nucleus of an atom. An experimentally determined number characteristic of a chemical element that represents the number of protons in the nucleus which in a neutral atom equals the number of electrons outside the nucleus and that determines the place of the element in the periodic table see Chemical Elements Table.
It is the weighted average of the masses of naturally-occurring isotopes. Atomic mass is also known as atomic weight. The mass number of an atom is the total number of nucleons ie.
What Is It Based On. Other atoms dont generally have round-number atomic masses for reasons that are a little beyond the scope of this article. 120 Zeilen Definition of Atomic Weight.
Atomic number and mass number - definition. Atomic number is the total number of protons present in the nucleus of an atom. Atomic mass is the weighted average mass of an atom of an element based on the relative natural abundance of that elements isotopes.
Prior to 1961 a unit of atomic weight was based on 116th 00625 of the weight of an oxygen atom. By definition an atom of carbon with six neutrons carbon-12 has an atomic mass of 12 amu. Atomic weight is the average mass of atoms of an element calculated using the relative abundance of isotopes in a naturally-occurring element.
The atomic mass of a single atom is simply its total mass and is typically expressed in atomic mass units or amu. A normal helium atom for example has two protons and two neutrons in its nucleus with two electrons in orbit. Difference Between Atomic Mass and Atomic Number.
Under what conditions are two atoms different isotopes of the same element. The dimensionless standard atomic weight is the weighted mean relative isotopic mass of a typical naturally-occurring mixture of isotopes. The atomic mass of atoms ions or atomic nuclei is slightly less than the sum of the masses of their constituent protons neutrons and electrons due to binding energy mass loss per E mc2.
If the reference standard is considered 1 u the atomic weight is the ratio of the average atomic mass to one unified mass unit 1 u. The sum of the mass number and the atomic number for an atom A-Z corresponds to the total number of subatomic particles present in the atom. Define atomic number and atomic mass number.
Each atom therefore can be assigned both an atomic number the number of protons equals the number of electrons and an atomic weight approximately equaling the number of protons plus the number of neutrons. Mass of protons AND of neutrons in an atomAtomic of protons in an atomIsotopes of an element Atoms of an element with the same atomic numbe. Difference Between Atomic Number and Atomic Weight Definition.
Atomic weight is given in amu atomic mass units. Definition of atomic number.