The two stable isotopes of helium are known as helium-3 and helium-4. One of the more prevalent uses of helium comes in the production of semiconductor chips.
![]() There S Still Plenty Of Room At The Bottom Isotopically Pure Materials For When Every Atom Counts Semiconductor Digest
There S Still Plenty Of Room At The Bottom Isotopically Pure Materials For When Every Atom Counts Semiconductor Digest
01785 grams per cubic centimeter.

Common isotopes of helium. There are two isotopes of Helium 3 He and 4 He. All the others are radioactive decaying very rapidly into other substances. Helium-3 3He and helium-4 4He are two isotopes of helium that appear in the.
In fact 9999986 of the helium found on Earth is 4 He. The vast majority of helium in the universe is 4 He and this is the same for our planet. Nov 12 2012 - There are eight known isotopes of helium.
Helium-4 the most common isotope is produced on Earth. Temperatures below a few Kelvin. Common Isotopes of Helium.
Helium-3 was discovered in 1939. Helium Isotopes Facts of the Cold Fusion Phenomenon. Helium has two stable isotopes 4He and 3He.
In nuclear physics helium ions or alpha particles serve as projectiles in bombarding heavy nuclei to produce energy or to obtain artificial radioisotopes. Other than protium ordinary hydrogen helium-3 is the only stable isotope of any element with more protons than neutrons. Helium-3 was discovered in 1939.
The known isotopes of helium contain from one to six neutrons so their mass numbers range from three to eight. A mixtureof the two isotopes separates spontaneously at temperatures below 08 K. Mobile phone computer and tablet chips.
Liquid helium is used in magnetic resonance imaging MRI equipment for the diagnosis of cancer and other soft-tissue diseases. Of these six isotopes only those with mass numbers of three helium-3 or 3 He and four helium-4 or 4 He are stable. Isotopes of helium 1 Although there are eight known isotopes of helium He standard atomic mass.
The most common of them is He-4. The liquids of both isotopes become superfluidsat low temperatures 4He below 217 K and. Other than protium ordinary hydrogen helium-3 is the only stable isotope of any element with more protons than neutrons.
Helium-3 is present on Earth only in trace amounts. 40026022 u only helium-3 3 He and helium-4 4 He are stable. Phones TVs computers tabletsif a device contains a chip it wouldnt be possible without the helium used at different stages in the production process.
Helium has seven known isotopes ranging from He-3 to He-9. Media in category Isotopes of helium The following 5 files are in this category out of 5 total. Helium-3 is a light stable isotope of helium with two protons and one neutron the most common isotope helium-4 having two protons and two neutrons in contrast.
Helium-3 3He see also helion is a light stable isotope of helium with two protons and one neutron the most common isotope helium-4 having two protons and two neutrons in contrast. Most of these isotopes have multiple decay schemes where the decay type depends on the overall energy of the nucleus and its total angular momentum quantum number. Neutron Scattering - Magnetic and Quantum Phenomena.
Helium-3 sorption systems work. 3750 per 1000 cubic feet. 4002602 atomic mass unit.
2 rânduri Helium has two isotopes but it consists almost entirely of He-4 with natural He only containing. Usually different isotopes of the same substance differ only in their mass. 2 Helium 3He 3016029 0000137 4He 4002603 99999863 3 Lithium 6Li 6015122 759 7Li 7016004 9241 4 Beryllium 9Be 9012182 100 5 Boron 10 B 10012937 199 11 B 11009305 801 6 Carbon 12 C 12000000 9893 13 C 13003355 107 14 C 14003242 7 Nitrogen 14 N 14003074 99632 15 N 15000109 0368.
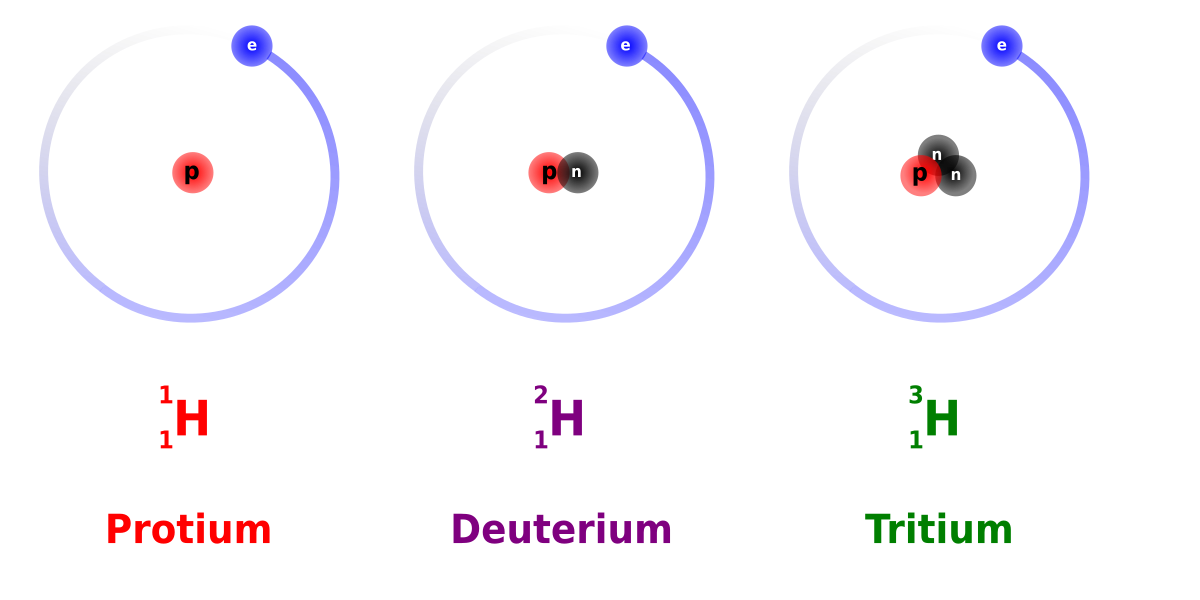 Isotopes Of Hydrogen Wikipedia
Isotopes Of Hydrogen Wikipedia
Introduction To Simple Atomic Structure
What Happens If You Take Away Neutrons From An Atom S Nucleus Quora
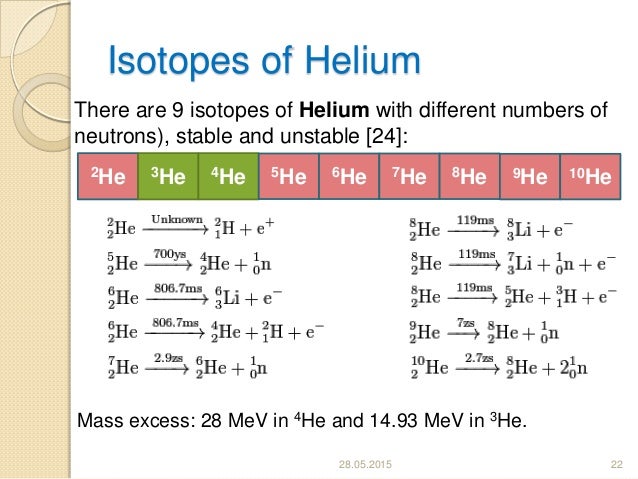 H E L I U M M O S T C O M M O N I S O T O P E Zonealarm Results
H E L I U M M O S T C O M M O N I S O T O P E Zonealarm Results
 Webelements Periodic Table Helium Isotope Data
Webelements Periodic Table Helium Isotope Data


/Helium_Tile-56a12a3d5f9b58b7d0bca98a.png)