Click to see full answer. This was later reversed by Carolus Linnaeus or Carl Linnaeus a Swedish botanist physician and zoologist after Anders died.
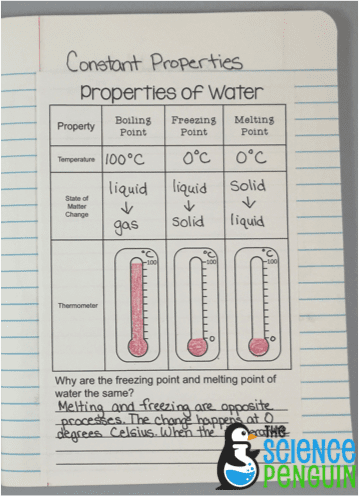 Constant Properties Of Water Boiling Point Melting Point And Freezing Point The Science Penguin
Constant Properties Of Water Boiling Point Melting Point And Freezing Point The Science Penguin
The red line shows the atmospheric pressure of the selected planet.

Boiling and freezing point of water. Water boils if the surrounding gases in the air have a high pressure. The boiling point of water varies with atmospheric pressure. A solution boils at a slightly higher temperature than the pure solvent.
Previously boiled water freezes faster than regular water Notwithstanding the previous explanation water at room temperature that was once boiled according to some experts should freeze faster because the dissolved oxygen has been removed. This week we will be identifying the boiling point of water and the freezing melting point of water. The change in the boiling point is calculated from.
As liquid matter boils it eventually vaporizes into a gas at the boiling point. Identify the boiling and freezingmelting points of water on the Celsius scale. For example the freezing point of pure water at standard atmospheric pressure or zero feet is 0C 32F while at 11km 6 miles above sea level it would only be 0001C higher.
At lower pressure or higher altitudes the boiling point is lower. On the Fahrenheit scale however freezing is 32 degrees and boiling 212. The freezing point describes the liquid to solid transition while the melting point is the temperature at which water goes from a solid ice to liquid water.
The freezing point or melting point of water is the temperature at which water changes phase from a liquid to a solid or vice versa. At 10000 feet above sea level the pressure of the atmosphere is only 526 mmHg. For example the boiling point of pure water at 10.
A mathematical equation is used to calculate the boiling point elevation or the freezing point depression. The boiling and freezing points of water enable the molecules to be very slow to boil or freeze this is important to the ecosystems living in water. A similar property of solutions is boiling point elevation.
Water has a boiling point of 100 C. The gas it usually turns into is called water vapor or steam. Textatm is 100texto textC while the boiling point of a 2.
Just as we can lower the boiling point we can also raise the boiling point of. Previously boiled water boils faster than regular water. Δ T b K b.
Under normal conditions when the pressure of the atmosphere is approximately 760 mmHg water boils at 100 o C. Because the freezing point of pure water is 0C the sucrose solution freezes at 068C. Scientists have found liquid water as cold as -40 degrees F in clouds and even cooled water down to -42 degrees F in the lab.
The boiling point elevation is the amount that the boiling point temperature increases compared to the original solvent. I cant tell you how surprised I was the first time I boiled water for my fifth graders. Not everyone has a vacuum chamber yet although they are getting quite inexpensive these days.
Boiling water is no big deal to us grown-ups because we see it almost every time we cook. The Fahrenheit scale defines the freezing point of water as 32F and the boiling point as 212F. The blue line shows the vapor pressure of water as a function of temperature.
The normal boiling point of water is 100 o C because this is the temperature at which the vapor pressure of water is 760 mmHg or 1 atm. The boiling point is the temperature at which the vapor pressure is equal to the atmospheric pressure. This Demonstration shows the boiling temperature of water on six planets all of which have different atmospheric pressures.
The meltingfreezing and boiling points change with pressure. The freezing point of water in degree Celsius is 0 C. Boiling point is the temperature at which something becomes a gas.
At at high altitudes the lower pressure makes the boiling point several degrees lower. This means that at 100 C water. At sea level pure water boils at 212 F 100C.
The Celsius scale sets the freezing point and boiling point of water at 0C and 100C respectively. Originally Anders Celsius assigned zero to stand for waters boiling point and 100 to stand for waters freezing point. Thats not always the case though.
The formula Degree Calsius C 27315 k 0 C 27315 27315k. In theory the two temperatures would be the same but. Then water gets to what is known as its boiling point.
The student is expected to. Raising the boiling point. If water was very easy to freeze or boil drastic changes in the environment and so in oceans.
For pure water the boiling point is 100 degrees Celsius 212 Fahrenheit at one atmosphere of pressure and the melting point is 0 degrees Celsius 32 degrees Fahrenheit at one atmosphere of pressure. Weve all been taught that water freezes at 32 degrees Fahrenheit 0 degrees Celsius 27315 Kelvin. Boiling Freezing and Pressure Altitude.
So 100ºC is now the boiling point of water while 0ºC is the freezing point of water. This may not be by a noticeable amount due to the volume change upon melting being much smaller than the volume change expansion when boiling.
/the-freezing-point-of-water-609418_FINAL-01f50f5f4f7d4a39854bebcc59df1aa4.gif) What Is The Freezing Point Of Water
What Is The Freezing Point Of Water
 What Is Water S Boiling Point In Fahrenheit The Millennial Mirror
What Is Water S Boiling Point In Fahrenheit The Millennial Mirror
 Boiling Melting Freezing Anchor Chart A Matter Of Temperature Science Anchor Charts Elementary Physical Science School Science Experiments
Boiling Melting Freezing Anchor Chart A Matter Of Temperature Science Anchor Charts Elementary Physical Science School Science Experiments
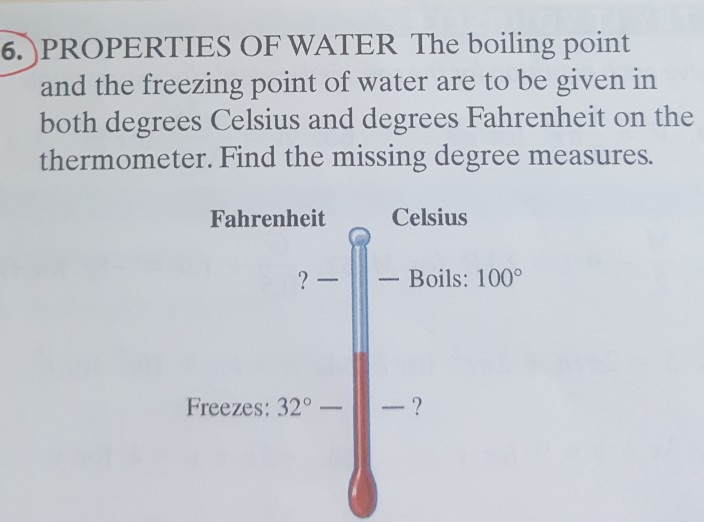 Solved 6 Properties Of Water The Boiling Point And The F Chegg Com
Solved 6 Properties Of Water The Boiling Point And The F Chegg Com
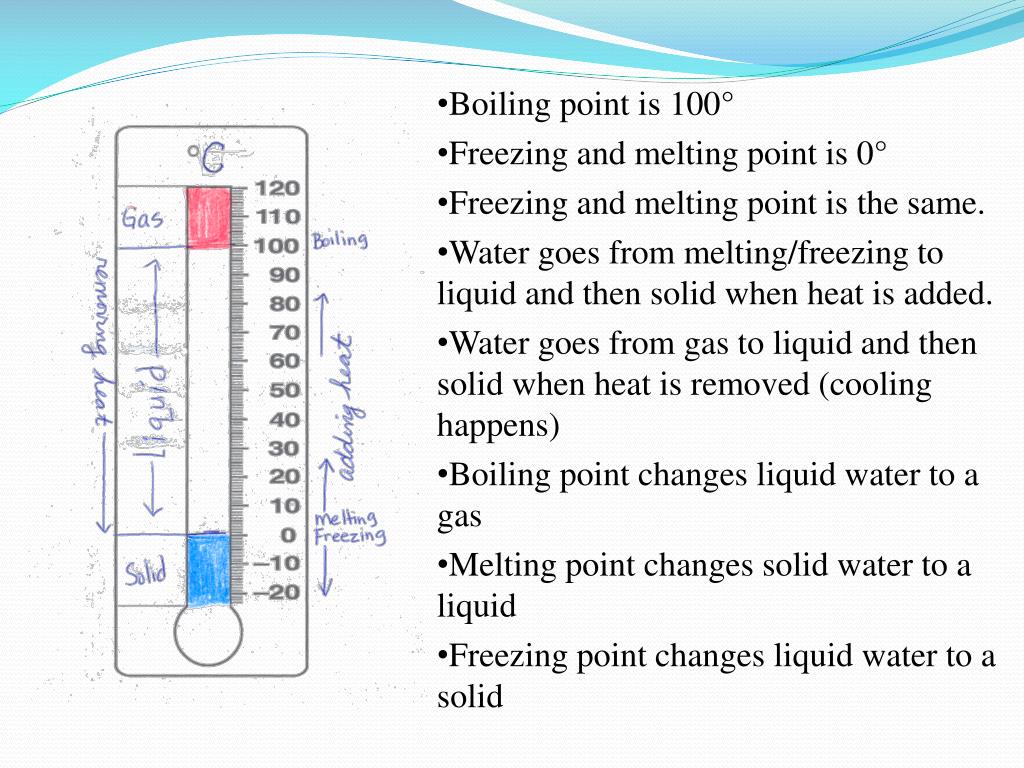 Ppt Physical Properties Of Water Boiling Point Melting Point And Freezing Point Powerpoint Presentation Id 3245093
Ppt Physical Properties Of Water Boiling Point Melting Point And Freezing Point Powerpoint Presentation Id 3245093
The Msds Hyperglossary Freezing Point
/the-freezing-point-of-water-609418_FINAL-01f50f5f4f7d4a39854bebcc59df1aa4.gif) What Is The Freezing Point Of Water
What Is The Freezing Point Of Water
 Boiling Point Freezing Melting Point Anchorchart Teaching Science Science Lessons Science Anchor Charts
Boiling Point Freezing Melting Point Anchorchart Teaching Science Science Lessons Science Anchor Charts