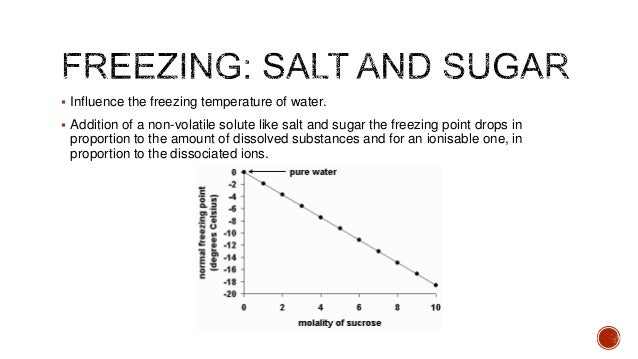
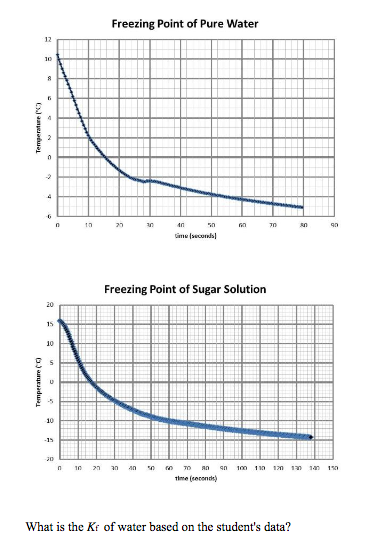
In the case of the salt water one molecule contains two elements - Sodium Na and Chloride Cl. Water freezes at 32 degrees Fahrenheit 0 degrees Celsius but when a solute such as sugar is added the freezing point changes.
So Sucrose has a relative freezing point depression of.

Freezing point of sugar water. Temperature measures how much. This will give you the total concentration of particles dissolved. Multiply the original molality text m of the solution by the number of particles formed when the solution dissolves.
Some examples for grams of sugar added to 1 liter water. There are many ways in which sugar molecules can fit into this jumble. A 5 ww solution of cane sugar in water has freezing point of 271 K.
This link says the standard 41 solution starts freezing at 26-27 degrees F. The change in freezing point compared to pure water is calculated by the difference between the average freezing point and the freezing point of water which is 0 ºC. So to calculate the freezing point depression do.
For water Kf is 186Kkgmol and the molar mass of sugar sucrose is 342gmol. Sugar molecules dont fit well in that array. Td 186 342 mass of sugar in g mass of water in kg.
The freezing point of 5 ww glucose in water is. The average of the freezing point of the sugar solution of 27 mol kg would be. The effective freezing point of sugar water is somewhat below 0 C.
The reason the salt and sugar lowers the freezing point and the water takes longer to freeze is because the salt or sugar molecules interfere with the attraction between the water molecules and therefore interfere with the webbing of the water molecules which prevents the water from turning into ice when the water temperature lowers even further it eventually reaches a temperature where the water. If you have added 1 molecule of sugar to water the water will freeze later at -18 C 2876 Fahrenheit. Popsicles are essentially just frozen sugar water -- theres no real reason why sugar water wont freeze.
For pure water this happens at 32 degrees Fahrenheit and unlike most other solids ice expands and is actually less dense than water. The degree to which a sugar lowers the freezing point of water is called the Relative Freezing Point Depression. Slowly freezing the water where the ice forms on one side and grows to another will probably separate the sugar out much better than quick freezing with many nucleation sites.
Note that CaCl 2 is substantially more effective at lowering the freezing point of water because its solutions contain three ions per formula unit. This will be determined by gathering data on the freezing point of several sugar and water solutions. Because the freezing point of pure water is 0C the actual freezing points of the solutions are 22C and 30C respectively.
A water molecule consists of one oxygen atom and two hydrogen atoms. The rate of freezing may play a very important role. Why Does Sugar Affect the Freezing Point of Water.
The sugar solution is a nonelectrolyte so its i value is only 1. Just like Relative Sweetness Relative Freezing Point Depression is measured against Sucrose. Why does that make it harder for the ice to form lowering the freezing point.
The sugar in a popsicle may be predominantly between less. A 6040 solution of ethylene glycol and water has a freezing point of about -45 C. The vant Hoff factor will have a direct relationship with the freezing point depression.
Via R 5 ww 514 51 fish 271 K C 5 ww out Hich - 1 2692K 2 271 K 3 272 K 4 2672K 5 none of these HT 7 11th. The temperature at which a liquid turns into a solid is known as its freezing point. The higher total concentration will result in a higher boiling point and a lower freezing point.
Plain water freezes at 32 degrees F but when sugar or salt or other solutes are dissolved in it the freezing point gets lower. 2925 2 27 ºC. Sugar water freezes faster than salt water because salt has more molecules than sugar.
The sugar solutions will then be compared to a sodium chloride solution on account. So when you freeze sugar water or juice the sugar stays behind in the liquid water as the ice forms. Heres a clue- it turns out that the type of molecule dissolved in the water doesnt make nearly as much difference for the freezing.
The sugar molecules prevent the water from making hydrogen bonds which are required for solidity and the water has to become even colder before it reaches its freezing point. The freezing point depression constant of water is what needs to be found. Gl Td 10 0054 50 027 500 27.
Normally water freezes at 32F however when a substance is added Normally water freezes at 32F however when a substance is added. Frozen water ice is a regular array of molecules. The sugar molecules will depress the water freezing point further.
A strong salt solution has a freezing point of about -21 C. Freezing happens when the molecules of a liquid get so cold that they slow down enough to hook onto each other forming a solid crystal.